
What if there were a magic substance or strategy that would actually slow down aging, allowing us to ease into our later years in generally excellent health? Turns out, there is, and it can help save the planet as well.
It’s not a drug — it’s an amino acid we should avoid — but the discovery of a new antibiotic helped us figure out how the system works.
Rapamycin: The Wonder Anti-Aging Drug?
Back in October of 1975, the Journal of Antibiotics (Vol 28, No 10) published an article titled, “Rapamycin, A New Fungal Antibiotic.” It noted that the compound was the metabolic product of a fungus found in the soil of Easter Island (known in the local language as Rapa Nui), and represented an exciting and newly-discovered streptomycete antibiotic whose “structure is still unknown.”
Rapamycin, it turns out, slows down aging by inhibiting a naturally-occurring enzyme in the human body that is known as mTOR, or the “mechanistic Target Of Rapamycin.”
This enzyme, mTOR, is, as the authors of a 2013 Nature article noted in the title of their piece, “A Key Modulator of Ageing and Age-Related Disease.” The authors noted in their abstract that finding drugs to “slow ageing” was inevitable, and:
“A leading target for such interventions is the nutrient response pathway defined by the mechanistic target of rapamycin (mTOR).”
They noted that when the mTOR enzyme was blocked in various animals, including primates like us, it both “extends lifespan” and “confers protection against a growing list of age-related pathologies.” If only we could take a pill — like the antibiotic Rapamycin — that blocks the mTOR enzyme’s metabolic pathway, the argument goes, we could all live longer and healthier lives.
The problem with taking regular doses of Rapamycin and substances derived from it to inhibit the action of the mTOR enzyme and thus slow ageing, however, is that these compounds are so new that human studies are not yet conclusive.
Rapamycin seems to stop — and, in some ways, even reverse — aging in mice and other animals, and there are multiple human clinical trials underway across the world, including some intended to end up before the FDA.
It’s the hot new drug for life-hackers who are trying to live longer, with concierge and online doctors prescribing 5 mg. of the drug one day every week. (There are message boards and groups across the internet by and for people taking this drug.)
How to Regulate Mtor Without Taking Drugs?
But for the average person, rapamycin isn’t an option, so what other ways can we alter the actions of the mTOR pathway in the human body to slow or reverse aging?
The mTOR pathway drives rapid growth throughout our first twenty to thirty years, but after that it continues to run at full speed, even though after roughly age 30 that process not only doesn’t produce “growth” but, instead, becomes a rapid form of aging.
Why doesn’t mTOR slow down as we age? Scientists believe it’s because throughout most of human evolutionary history, aging beyond the 40s was pretty much non-existent.
But now that modern hygiene and medicine have extended our life spans into an average of the 70 to 80 years range, the holy grail of anti-aging efforts worldwide has been trying to figure out how to inhibit the mTOR enzyme after the first 20-30 or so years of rapid growth and muscle development.
As Mikhail V. Blagosklonny wrote for the journal Cell Cycle:
“[T]he traditional analogy of an aging organism with a rusting (albeit self-repairing) car is misleading. The true analogy is a speeding car that enters a low-speed zone [after age 30] and damages itself because it does not and cannot slow down.”
While there isn’t an FDA-approved drug that we can take today to slow down aging, there is a change we can make to our diet that measurably and reliably slows down the mTOR enzyme’s aging pathway. And that dietary change has an evolutionary history, too.
How Feast And Famine Produces A Prolonged Life
Throughout human history, pretty much wherever humans lived, there were periodic times of — literally — feast and famine. Three researchers from the European Institute of Oncology noted in the title of a 2012 article in Frontiers of Physiology how “caloric restriction” produces “a healthy and prolonged life.”
The opening two sentences of the article summarize its thrust:
“Over the last several years, new evidence has kept pouring in about the remarkable effect of caloric restriction on the conspicuous bedfellows: aging and cancer. Through the use of various animal models, it is now well established that by reducing calorie intake one can not only increase life span but, also, lower the risk of various age-related diseases such as cancer.”
Does that mean that we all need to fast a few days a week, or a few weeks a year, replicating, in part, the world we lived in before the advent of modern agriculture? Or adopt the now-popular 5:2 diet or something similar, where we eat fewer than 500 calories for two days every week, while eating our normal diet the other five?
While those types of caloric restriction certainly have been proven to slow down aging in other mammals, and appear to do so with human populations who’ve been exposed to famine or had anorexic periods in their lives, such a drastic measure may not be necessary to extend both lifespan and quality of life as we age. And it all comes back to the master enzyme pathway of rapid growth and rapid aging: mTOR.
mTOR Is The Master Enzyme Controlling Aging
Nature’s journal Molecular Cell Biology published a remarkable article in January, 2011, titled mTOR: From Growth Signal Integration to Cancer, Diabetes and Aging. In the clinical language of their science, the authors wrote:
“The mechanistic target of rapamycin (mTOR) coordinates eukaryotic cell growth and metabolism with environmental inputs, including nutrients and growth factors. Extensive research over the past two decades has established a central role for mTOR in regulating many fundamental cell processes, from protein synthesis to autophagy, and deregulated mTOR signaling is implicated in the progression of cancer and diabetes, as well as the aging process.”
In other words, mTOR is a master enzyme pathway that “coordinates” both how cells metabolize nutrients, and how fast they grow as a result. It regulates everything from the ability of cells to make proteins for other parts of the body to the process of destroying old, diseased, or damaged cells (autophagy) that could otherwise turn cancerous or problematic.
Even more significantly, when mTOR isn’t controlled or runs in a “deregulated” fashion, aging speeds up and people move faster towards cancer and diabetes.
The article also notes that outside of Rapamycin, no individual compound has yet been found to down-regulate or slow down the mTOR pathway, except caloric restriction.
You Don’t Need To Starve: Just Avoid One Particular Type Of Food
So, what is it about caloric restriction that regulates the mTOR pathway so effectively? Turns out, it’s not the simple restriction or reduction of calories, although that works.
But the reason it works is because when people eat fewer calories — they eat less food — they’re also consuming less protein. And protein, it appears, is what’s actually hitting the mTOR pathway and speeding up (via lots of dietary protein) or slowing down (with less dietary protein) aging and the chance of getting cancer and diabetes.
A study published in the journal Aging Cell in October of 2008 noted that while caloric restriction “protects against cancer and slows aging in rodents,” that, prior to the publication of this study, “long-term effects of caloric restriction…in humans are unknown.”
They then report that in protein-restricted humans, who were able to eat all the calories they wanted, the result was a significant reduction of the hormones and metabolic pathways that lead to aging and metabolic diseases like diabetes and cancer.
They ended their abstract with:
“[O]ur data provide evidence that protein intake is a key determinant of circulating IGF-1 levels in humans, and suggest that reduced protein intake may become an important component of anticancer and anti-aging dietary interventions.”
Protein reduction, in fact, not only down-regulates the activity of the mTOR pathway, but it does the same for IGF1, another compound that is associated with aging and metabolic diseases, as an article titled Extending Healthy Life Span—From Yeast to Humans published in Science in 2010 lays out clearly.
So, it turns out it’s not restricting calories that produces an increase in human longevity (although binging on calories regularly isn’t healthy, either), but restricting protein.
But Are There Good And Bad Proteins For Aging?
Taking that to the next step, what if it wasn’t even the protein, but one of the specific amino acids that make up the protein that was producing — when reduced — this aging-slowing outcome?
An article published in the journal Trends in Cell Biology points out that, “Withdrawal of [the amino acid] leucine has been shown to be nearly as effective in down-regulation of mTOR1C signaling as withdrawal of all amino acids.”
Leucine — The Amino Acid That Ages You
Leucine, it turns out, is an amino acid that is found in very low concentrations in edible plants and plant-based products, and in very high concentrations in meats and other food products derived from animals, ranging from mammals (and dairy) to birds (and eggs) to fish.
Researchers in Germany found that feeding human children infant formulas fortified with cow’s milk — which is much higher in leucine than is human mother’s milk — can produce obesity and even diabetes in children as young as two years old. Leucine, they noted, was the key to down-regulating the IGF and mTOR pathways.
“In general,” the authors noted, “lower leucine levels are only reached by restriction of animal proteins.”
“Whey proteins” from cow’s milk, they noted, “…contain the highest amount of leucine (14%), followed by casein (10%), the major protein constituent of cow milk and cheese.” Flesh meats are just slightly lower than dairy products.
Reducing leucine slows down aging and cancer, and the easiest and most painless way to reduce leucine is by eliminating all or most animal products from your diet.
And the differences in leucine levels are huge between animal and plant-based foods:
“Just 100 grams (about 3.5 ounces) of Gouda cheese contains 2400 milligrams of leucine,” they wrote, whereas hitting that same leucine level with vegetables would require eating “4.2 kg [over 9 pounds) white cabbage or 100 apples.”
They concluded that section noting, “These calculations exemplify the extreme differences in leucine amounts provided by an animal meat/dairy protein-based diet in comparison to a vegetarian or vegan diet. Thus, the increased consumption of meat and dairy proteins provide excessive amounts of leucine for mTORC1 activation.”
This entire long and winding road of science and logic leads to a single inescapable point. Reducing leucine slows down aging and cancer, and the easiest and most painless way to reduce leucine is by eliminating all or most animal products from your diet.
Living Longer Through Leucine Restriction Also Helps Save Our World
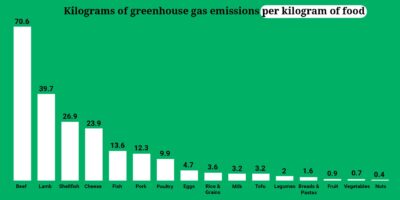
The bonus here is that a plant-based diet is better for our planet. Animal-based agriculture is among the top five contributors to climate-changing greenhouse gasses worldwide, because first we have to grow the grains and vegetables that we then feed to the animals we ultimately eat.
The entire process is incredibly inefficient, as you can see in this UN-provided chart:
A single pound of feedlot-produced beef requires the nutrient base of 35 pounds of topsoil (to produce 12 pounds of grain) and about 2500 gallons of water.
While different vegetable, grain, seed, nut, and fruit crops consume or require different amounts of topsoil and water to produce a pound of edible plant-based food, the amounts are radically smaller than what’s necessary to produce a pound of animal flesh, eggs, or dairy.
So, save the world and lengthen your own life: cut back on animal products as much as you can and you’ll become part of the (long-lived) solution rather than the problem.
Original article here






















Sorry, the comment form is closed at this time.